The chemical reaction is a process in which a chemical substance transforms into a set of another chemical substance. When two or more chemicals react with each other they convert into another chemical. A simple chemical reaction is as represented below.
![]()
Example: Rust is formed when iron undergoes a chemical reaction with oxygen.
In a combustion reaction, carbon reacts with oxygen to form carbon dioxide.
![]()
Rate of reaction or reaction rate is defined as the rate at which chemical reaction takes place. It is the rate at which the chemical reactants are converted into products. It gives the time frame in which the reaction is completed.
Example: the rate of reaction involved in the combustion of cellulose in fire takes place in a fraction of a second. Hence the rate of reaction involved is instantaneous.
Example 2: it takes years for iron to undergo oxidative rusting. Hence the rate of reaction involved is high.
As we know that the rate of reaction varies from reaction to reaction. Some reactions are instantaneous and some take years to attain equilibrium. The speed at which a reaction takes place is known as the rate of a reaction.
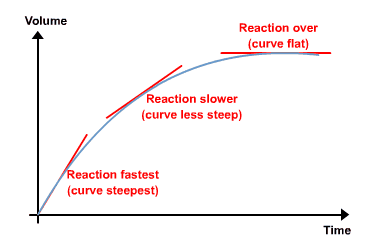
Below graph clearly explains the three stages in which the reactions take place. The reactions which take place at a fast pace is indicated at the starting of the curve.
The reaction which takes place slowly is indicated in the mid-range of the curve.
The reaction which is completed or achieved the equilibrium state is mentioned over the flat curve.
Rate of reaction is affected by many factors like:
- Nature of the reaction and the compounds involved.
- The concentration of the reactants
- Pressure factor
- Temperature
- Solvent and catalyst involved
Let us know about one more type of reaction – The Wittig reaction
The Wittig reaction is also known as Wittig olefination. It is a type of chemical reaction in which aldehyde or ketone in the presence of Wittig Reagent (triphenyl phosphonium ylide) produces an alkene together with triphenylphosphine oxide.
Wittig reaction is as represented below.
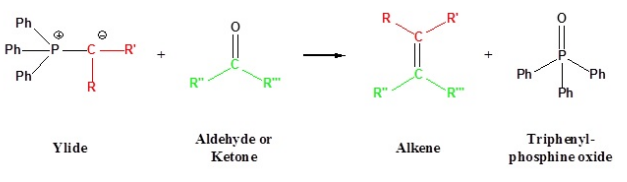
Wittig reaction is the contribution of the German chemist Georg Wittig and is named after him. Georg Wittig has also awarded the highest honor, the Nobel Prize in Chemistry for his outstanding contribution of discovering Wittig reaction in the year 1979. This reaction is mainly used for the manufacturing of alkenes using organic synthesis method.
Wittig reaction is the most common form of reactions that are used to combine aldehydes and ketones to form triphenylphosphonium ylides. Nature of the ylide is easily predicted based on the double bond geometry that is found in the reaction involving aldehydes.
To know more about the rate of reaction and Wittig reaction, visit BYJU’S!!
